India is expected to save a lot of foreign exchange with a team of researchers developing a new technique that promises to help in more effective production of a chemical molecule called 1,4 hydroquinone, which is used as an intermediate in the manufacture of food preservatives, pharmaceuticals, dyes, and polymers. India currently imports 1,4 hydroquinone at high costs.
1,4-hydroquinone is produced by oxidising another chemical called phenol. Conventionally, phenol oxidation is carried out by chemical methods using catalysts involving precious metals, metal oxides, and enzymes along with hazardous oxidants. But these methods suffer from many disadvantages, including incomplete conversion of starting material and lack of product selectivity, along with environmental hazards.
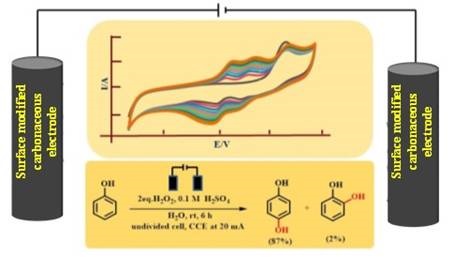
In the new study, researchers at the Centre for Nano and Soft Matter Sciences, an autonomous research institute under the Department of Science and Technology (DST), Government of India, and CSIR-National Chemical Laboratory have developed a technique based on electrolysis that promises to oxidise phenol to 1,4 hydroquinone more effectively.
India is expected to save a lot of foreign exchange with a team of researchers developing a new technique that promises to help in more effective production of a chemical molecule called 1,4 hydroquinone, which is used as an intermediate in the manufacture of food preservatives, pharmaceuticals, dyes, and polymers. India currently imports 1,4 hydroquinone at high costs.
Electrochemical organic transformations are being looked at with great interest in recent times because of various economic and environmental advantages they offer over conventional chemical methods. These transformations are typically carried out in an aqueous medium by just passing electricity through the substrate (in this case phenol). Consequently, no environmentally hazardous oxidants/reductants are involved in this process.
However, there are several practical issues, especially concerning phenol oxidation. For example, the conventional metal-based electrodes could not be used for this transformation as they start losing activity over time due to the adsorption of the oxidised products on their surfaces. Furthermore, many times they lead to over oxidation of phenol resulting in a lack of product selectivity and unwanted product formation such as tar. Additionally, some of the electrodes also suffer from issues like physical stability and durability of the electrodes with time.
In their study, the NCL and CeNS researchers have found that all these difficulties could be overcome by using electrodes having disordered graphene-like structures with the right number of oxygen-bearing surface functional groups such as hydroxyl, carboxyl, and carbonyl groups. The surface modification was done by electrochemical treatment of the electrode in an acidic environment. The researchers have established the optimum conditions for this surface modification. They could achieve a 99 percent conversion of phenol with 87% selectivity to 1,4-hydroquinone.
Announcing the new work, an official press release said that the researchers are currently looking at other industrially relevant processes that could be accomplished by such environmentally benign electro-organic transformations.
India Science Wire
ISW/SP/DST-CSIR/IMPORT REDUCTION/04/01/2022